Somatic hybridization is the technique of hybrid production through the fusion of isolated somatic(body) protoplasts under in vitro conditions and subsequent development of their product (heterokaryon) to a hybrid plant.
This involves –
- fusion of protoplast
- selection of hybrid cells
- Identification of hybrid plants
Table of Contents
A. Fusion of protoplast cells –
- Mixing of protoplast of two different genome
- it can be achieved by
- Spontaneous fusion
- Process in integral to plant development. eg. egg fertiliation
- Observed when protoplast isolated cell from callus
- Do not generate into whole plant but few division.
- Induced fusion
- Fusion can be achieved by adding suitable fusogen e.g. NaNO3.
- Various types of fusogen are used for fusion in different mathod
- Some popular method of fusion are as –
- Treatment with NaNO3
- Ca2+ at High pH
- PEG method
- Electrofusion
1. Treatment with NaNO3 –
1ST Rerport by power et al., 1970. In this method NaNO3 is used as Fusogen (an agent which induce fusion of cells).
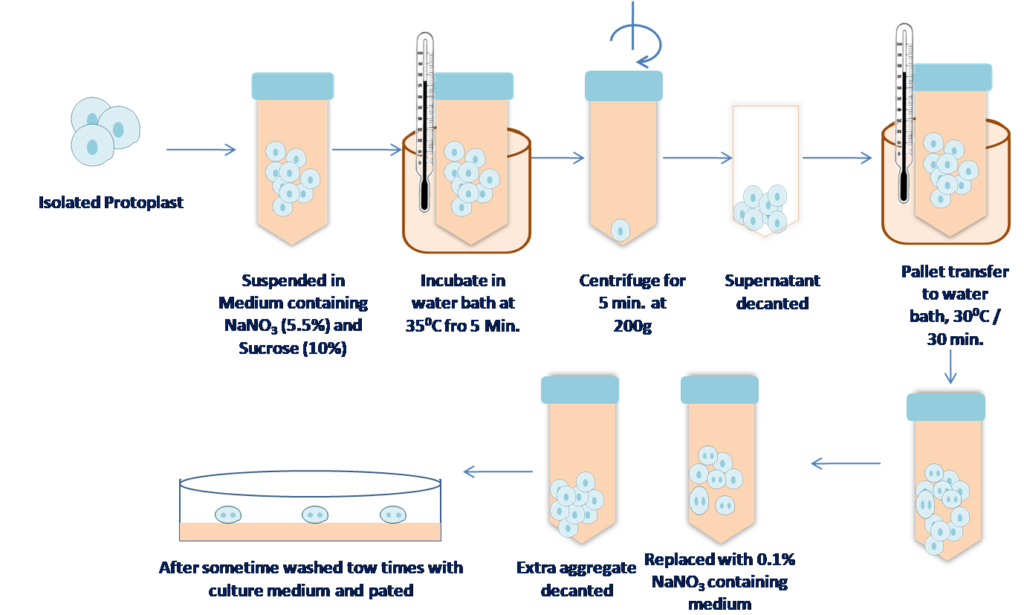
Figure 2 Fusion of protoplast by NaNO3 treatment method
2. Ca2+ at high pH :
In this method Fusion are induced at high calcium and pH condition. Mannitol is used as fusogen.
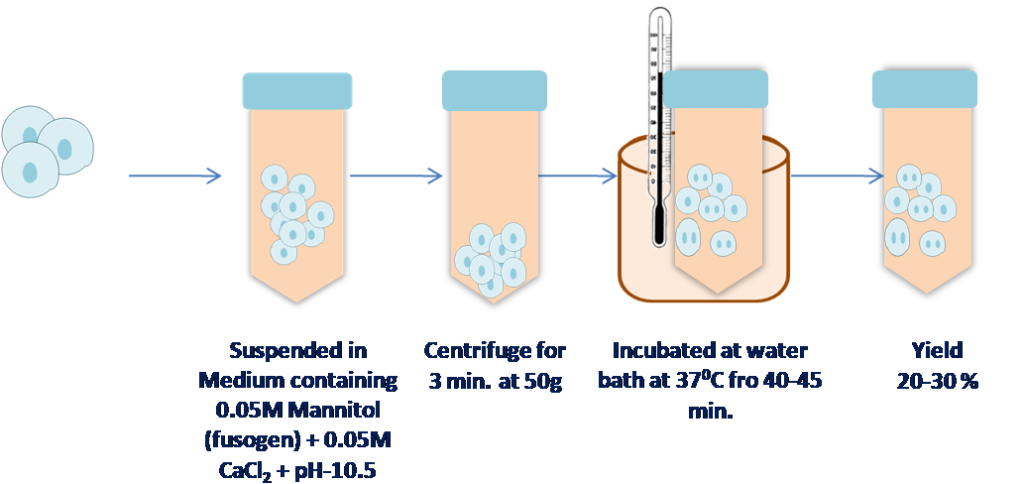
Figure 3 Ca++ at High pH treatment for protoplast fusion
3. Polyethylene Glycol (PEG) method :
- PEG enhace agglutination and fusion of protoplast.
- It is the most successful method for the protoplast fusion.
- has High frequency heterokaryon formation
- low toxicity
- formation of binuclear heterokaryon
- fusion is non-specific
- useful for interspecific, intergenic and interkindgom fusion.
- PEG with high pH /Ca++ reported most effective in enhancing fusion frequency & survivability of protoplast.
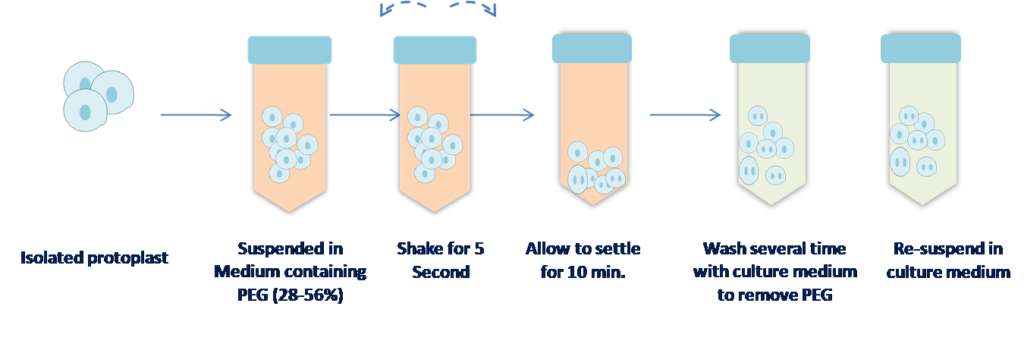
Figure 4 PEG method of protoplast fusion.
4. Electrofusion :
Protoplast are placed into a small culture cell containing electrode and a potential difference is applied due to which protoplast line up between the electrode.
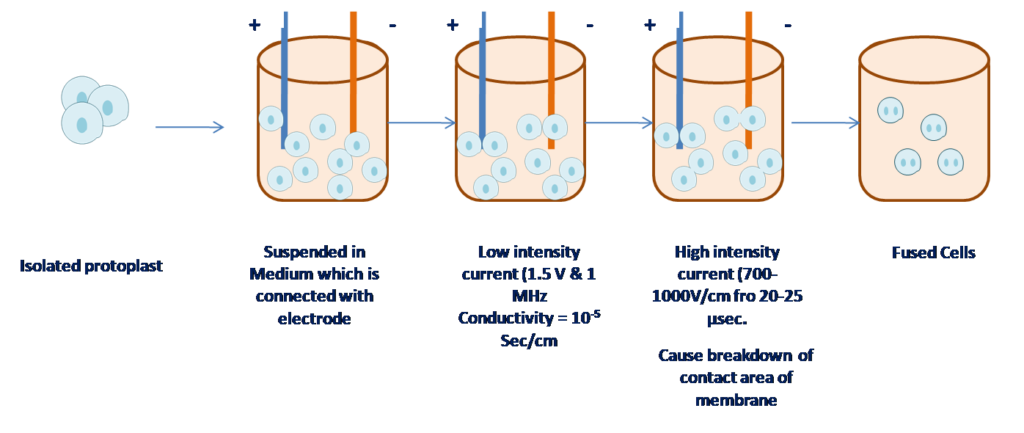
Figure 5 Electrofusion method for protoplast
- Advantage: Simple, Quick, Efficient, low toxic
- Disadvantage: Require specialized equipment.
B. Identification and selection of hybrid cells-
After fusion of protoplast as we seen from above. The medium contain mix population of cell means it contain fused cell (homokaryon cell or heterokaryon cells) and non-fused cells ( Parental cells) or cells with variety of other nuclear or cytoplasmic combination.
so we have to select our desired hybrid (usually heterokaryon) from these mixture and culture it, to get hybrid plant.
various method can be used to select hybrid –
- Chlorophyll deficiency complementation
- Auxotroph complementation
- Complementation of resistance marker
Chlorophylleficiency complentation :
Chlorophyll deficiency complementation is a concept related to the restoration of normal chlorophyll biosynthesis in plants or photosynthetic organisms that have a mutation preventing them from producing chlorophyll, a pigment essential for photosynthesis. Such mutations result in chlorophyll-deficient mutants, often exhibiting pale or albino leaves due to their inability to synthesize chlorophyll.
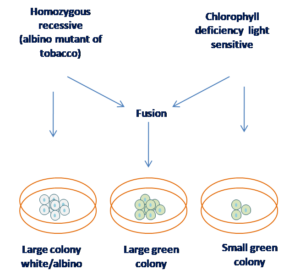
Figure 6 Selection of hybrid cells by chlorophill deficient method
Auxotroph complementation –
Auxotroph complementation is a genetic technique used to identify and study genes by exploiting the metabolic deficiencies (auxotrophies) of certain organisms. An auxotroph is a mutant organism (often a bacterium or yeast) that cannot synthesize a particular compound required for its growth, due to a gene mutation. This contrasts with a prototroph, which has no such deficiency and can grow in minimal media.
When two auxotrophic strains with different mutations are combined, one strain can supply the metabolic function that the other lacks, allowing both to grow in conditions where neither could grow alone.
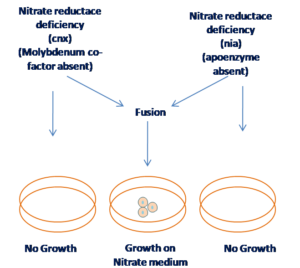
Figure 7 Auxotrophic selection of hybrid cells
Complementation of resistance markers
In this method, each parental protoplast carries a different resistance marker gene—one parent might be resistant to an antibiotic (e.g., actinomycin D) etc.
Complementation of resistance markers occurs when the fused hybrid protoplasts inherit both resistance genes, allowing them to survive in the selective medium.
Common Resistance Markers:
- Antibiotic resistance: Genes conferring resistance to antibiotics such as kanamycin, hygromycin, or streptomycin are frequently used.
- Herbicide resistance: Resistance to herbicides like phosphinothricin (Basta) or glyphosate can also be used as a selection marker.
- Metabolic complementation: In some cases, complementation of auxotrophic traits (like the inability to synthesize a specific amino acid) can be used, where one protoplast provides a missing metabolic function for the hybrid.
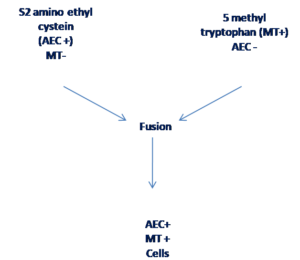
Figure 8 Selection of hybrid cells by various markers
Verification of hybrid plant
Verification of hybrid plants generated after protoplast fusion is crucial to confirm that the resulting plants are true hybrids and have combined the desired characteristics of both parent plants. Several methods are employed to verify hybrid plants at different levels, including morphological, biochemical, cytogenetic, and molecular approaches. Below are the commonly used verification techniques:
1. Morphology-Based Verification:
- Visual examination of plant traits such as leaf shape, flower color, plant height, and overall growth habit can provide initial evidence of hybridization.
- Intermediate Traits: True hybrids often show intermediate characteristics between the two parent plants.
- Phenotypic Evaluation: Specific traits of interest, such as disease resistance, drought tolerance, or yield, can be evaluated to ensure the hybrid exhibits the expected improved characteristics.
- Limitations: Morphological verification alone is not always reliable because environmental factors can also influence traits, and hybrids may display traits that are not immediately distinguishable.
2. Isoenzyme Analysis:
- Isoenzymes (or isozymes) are different forms of an enzyme that catalyze the same reaction but differ slightly in structure. Since different species often have distinct isoenzyme patterns, these can be used to confirm hybrid status.
- Hybrid Detection: The hybrid will display isoenzyme bands corresponding to both parent plants, confirming the presence of genetic material from both.
- Advantages: Isoenzyme analysis is relatively simple and inexpensive, providing a quick way to check for genetic exchange in hybrids.
- Limitations: It may not be able to detect small-scale genetic changes or recombination events and can miss some hybrids, especially if isoenzyme markers are not sufficiently informative for the species being studied.
3. Chromosomal Constitution (Cytogenetic Analysis):
- Chromosome Counting: Hybrid plants often exhibit a different chromosome number from either parent or an altered chromosome configuration, making chromosome counting a key method for confirming hybridity.
- Chromosome Staining: Techniques like Giemsa staining or fluorescence in situ hybridization (FISH) can reveal specific chromosomal patterns.
- Chromosome Pairing in Meiosis: Observation of chromosome pairing during meiosis can indicate whether the chromosomes from the two parents are pairing properly, which is a hallmark of successful hybridization.
- Ploidy Analysis: Hybrids can be diploid, triploid, or tetraploid, depending on the parental contributions. Ploidy can be assessed using flow cytometry or by directly counting chromosomes in mitotic or meiotic cells.
- Limitation: Chromosome analysis is time-consuming and requires expertise in cytogenetics.
4. Molecular Techniques:
- Molecular techniques provide the most definitive verification of hybrid plants, as they can directly analyze the plant’s DNA. Common methods include:
- Restriction Fragment Length Polymorphism (RFLP): This method compares the DNA fragment patterns of the hybrid and parent plants by cutting the DNA with restriction enzymes. Hybrids should show a combination of bands from both parents.
- Random Amplified Polymorphic DNA (RAPD): RAPD uses random primers to amplify segments of the genome. A hybrid will show a combination of RAPD bands from both parent plants.
- Amplified Fragment Length Polymorphism (AFLP): A more specific and sensitive technique that amplifies DNA fragments after digestion with restriction enzymes. Hybrids will exhibit band patterns from both parents.
- Simple Sequence Repeat (SSR) or Microsatellite Analysis: SSRs are short, repetitive DNA sequences scattered throughout the genome. SSR markers are used to verify hybrids by comparing the specific alleles from both parents. The hybrid should inherit alleles from both parents, giving an informative profile.
- Molecular Fingerprinting: Techniques such as DNA barcoding or genotyping-by-sequencing (GBS) can generate detailed genetic profiles to confirm hybridity.
- Polymerase Chain Reaction (PCR)-Based Markers: PCR-based markers like sequence-tagged sites (STS) or cleaved amplified polymorphic sequences (CAPS) can be used to identify specific genes or genomic regions from both parents in the hybrid.
- Limitations: These molecular methods can be technically challenging and require access to specialized equipment and reagents, but they offer the highest precision in verifying hybrid plants.
5. Genetic Character Verification:
- Inheritance Studies: The hybrid’s progeny can be evaluated to see if the desired traits are stably inherited in subsequent generations. This is especially important for traits like resistance to diseases, herbicides, or environmental stressors.
- Quantitative Trait Analysis: For complex traits, genetic verification can be done by analyzing the segregation patterns of the traits in hybrid progeny, which involves measuring the extent of trait expression and determining whether it matches expected Mendelian ratios.
Applications of Somatic Hybridization:
Development of Disease-Resistant Plants:
- Somatic hybridization enables the transfer of disease resistance genes from wild or related species to cultivated crops
- This approach is particularly valuable for crops with limited natural resistance or for diseases that cannot be effectively managed using traditional breeding methods.
Cytoplasmic Male Sterility (CMS) for Hybrid Seed Production:
- Somatic hybridization can be used to create cybrids—plants that inherit nuclear DNA from one parent and cytoplasmic DNA (mitochondria and chloroplasts) from another. This is essential for developing cytoplasmic male sterility, which is widely used in the production of hybrid seeds.
Overcoming Sexual Incompatibility:
- Somatic hybridization allows hybridization between species that are sexually incompatible or sterile. For example:
- Potato + Tomato (Pomato): Although potatoes and tomatoes belong to the same family (Solanaceae), they cannot be easily crossbred. Somatic hybridization has been used to produce “pomato” plants, though they are more useful for research than for commercial production.
Transfer of Abiotic Stress Resistance:
- Hybridization through protoplast fusion has allowed for the transfer of genes conferring resistance to abiotic stresses like drought, salinity, and extreme temperatures from stress-tolerant wild species to sensitive crop varieties.
Improvement of Crop Quality Traits:
- Somatic hybridization can combine desirable traits from different species or varieties, leading to improved nutritional, flavor, or storage qualities.
Incorporation of Novel Traits from Distant Relatives:
- Somatic hybridization enables the introduction of traits from distant or wild relatives that may carry important agricultural traits, such as improved tolerance to pests or diseases.
Enhancement of Hybrid Vigor (Heterosis):
- Somatic hybridization can enhance hybrid vigor (heterosis), resulting in improved growth rates, yield, and adaptability. This is particularly useful in breeding programs aimed at improving yield in crops like rice, wheat, and maize.
Creation of New Crops:
- In some cases, somatic hybridization has resulted in the creation of entirely new plant types with unique characteristics.
- Pomato (Potato + Tomato): While not widely used in agriculture, the fusion of potato and tomato plants into a single organism (producing both tomatoes and potatoes) is an example of novel plant creation through somatic hybridization.
- Triticale (Wheat + Rye): Triticale, a hybrid between wheat (Triticum) and rye (Secale), is a successful somatic hybrid used as a high-yielding, stress-tolerant grain crop.
Hybridization of Forest Trees:
- Somatic hybridization has been applied to improve commercially important forest trees by combining traits such as faster growth, better wood quality, and resistance to diseases.
Restoration of Fertility in Hybrids:
- Somatic hybridization can sometimes restore fertility in interspecific or intergeneric hybrids that are otherwise sterile. This is particularly useful in overcoming fertility barriers between species or varieties.
- Brassica: In Brassica rapa, somatic hybridization has been used to restore fertility in hybrids between B. rapa and other Brassica species.
Advantages of Somatic Hybridization:
- Overcomes sexual incompatibility barriers: Useful in species where traditional cross-breeding is not possible.
- Combines desirable traits from different species: Especially important for traits like disease resistance or abiotic stress tolerance.
- Expands genetic diversity: Incorporates novel genes from wild or distantly related species into cultivated crops.
- Rapid introgression of traits: Somatic hybrids can rapidly introduce specific traits without the need for several generations of breeding.
Limitations:
- Somaclonal variation: Tissue culture techniques used in somatic hybridization can sometimes introduce unwanted genetic variations.
- Difficulty in regenerating hybrids: Not all protoplast fusions successfully regenerate into viable hybrid plants.
- Limited success in monocots: While somatic hybridization has been highly successful in dicots (e.g., Brassica, tobacco), it has been more challenging in monocots like cereals.