Monoclonal antibodies (mAbs) are highly specific and uniform antibodies that are produced from a single clone of plasma (B) cells against a single epitope of an antigen.”
It offers a consistent and reproducible reagent for various diagnostic applications. Due to their precision, monoclonal antibodies have revolutionized diagnostic methods in medicine, biotechnology, and research.
Diagnosis of disease is complex, requiring clinicians to interpret symptoms and observations to determine the underlying cause, predict effective therapies, and monitor treatment outcomes.
Laboratory tests that identify infectious agents, abnormal cells, or disease-related molecules in body fluids or tissues provide crucial information for diagnosis, differential diagnosis, and therapy selection.
Antibodies, because of their exquisite specificity, are particularly useful reagents in this context, and monoclonal antibodies generally show superior specificity compared with polyclonal mixtures of antibodies.
Monoclonal antibodies are widely used in disease diagnosis across diagnostic labs, doctors’ offices, and field settings
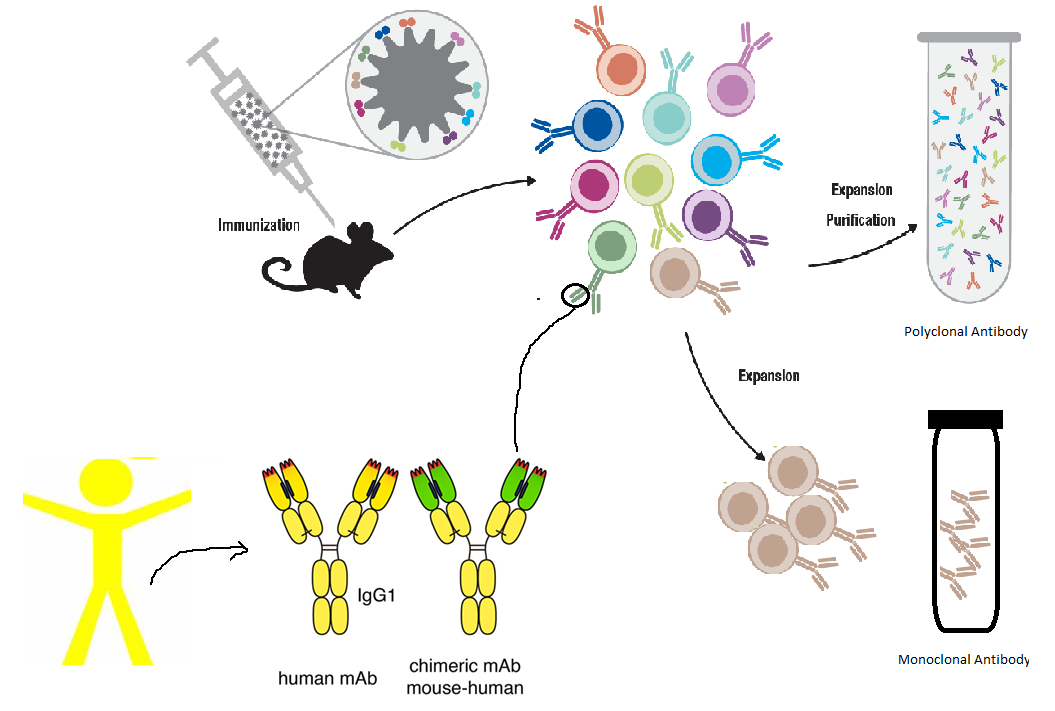
Table of Contents
Early History
Early researchers relied on polyclonal antibodies, which are produced by multiple B cells and recognize various parts of an antigen. While useful, polyclonal antibodies had significant limitations:
- Variability
- Non-specific binding:
- Short-term solution
The scientific world needed a solution that provided uniformity, specificity, and reliability.
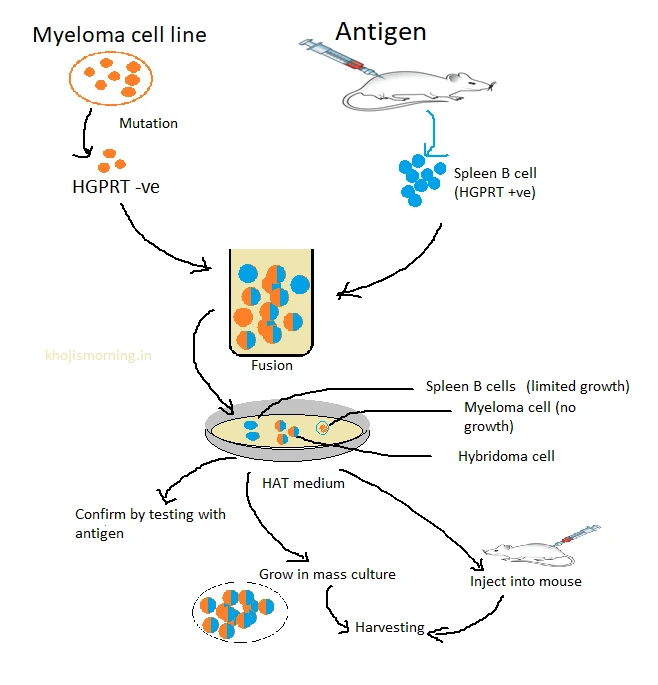
In a technique called hybridoma technology was Developed by G.Kohler and C.Milstein and monoclonal antibody production became possible using this technology, they received Nobel Prize for their groundbreaking work.
This technology revolutionized diagnostics, making it possible to produce antibodies from a single clone of B cells that bind to only one specific part (epitope) of an antigen.
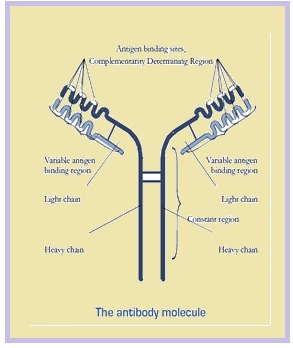
Structure of monoclonal antibody
- An antibody, also known as an immunoglobulin, is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses.
- Antibodies are large heterodimeric molecules and are composed of two types of polypeptide chains, called the heavy and the light chain. The two types of light chains are kappa and lambda.
- Each tip of the “Y” of an antibody contains a paratope that is specific for one particular epitope on an antigen.
- Monoclonal antibodies are antibodies that are made by identical immune cells that are all clones of a unique parent cell.
- Monoclonal antibodies can have monovalent affinity.
Production Process of Monoclonal Antibodies as Diagnostic Reagents
- Immunization: A mouse (or other animal) is immunized with an antigen of interest.
- Fusion: B cells from the immunized animal are fused with myeloma cells to form hybridomas (hybrid cells with the properties of both parent cells).
- Selection: The hybridomas are selected for the production of antibodies specific to the antigen.
- Cloning: The hybridomas are cloned to obtain a pure population of cells that produce the desired monoclonal antibody.
- Purification: The mAbs are harvested and purified for use in diagnostics.
Applications of Monoclonal Antibodies as Diagnostic Reagents
Monoclonal antibodies are versatile tools in a variety of diagnostic techniques due to their high specificity, reproducibility, and ability to bind to unique markers.
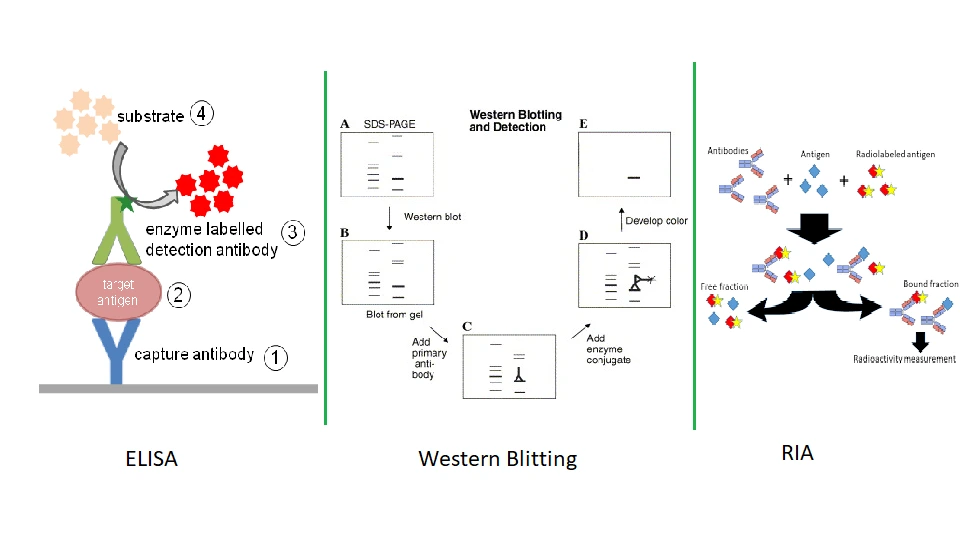
- Immunoassays: mAbs are widely used in assays that detect and quantify antigens in biological samples. Common immunoassay formats include:
- Enzyme-Linked Immunosorbent Assay (ELISA): Detects the presence of antigens (or antibodies) in a sample by using an enzyme-linked monoclonal antibody to produce a color change as a readout.
- Western Blotting: mAbs can be used to identify specific proteins in complex mixtures based on their molecular weight.
- Radioimmunoassay (RIA): Uses radiolabeled mAbs to detect antigens in the sample.
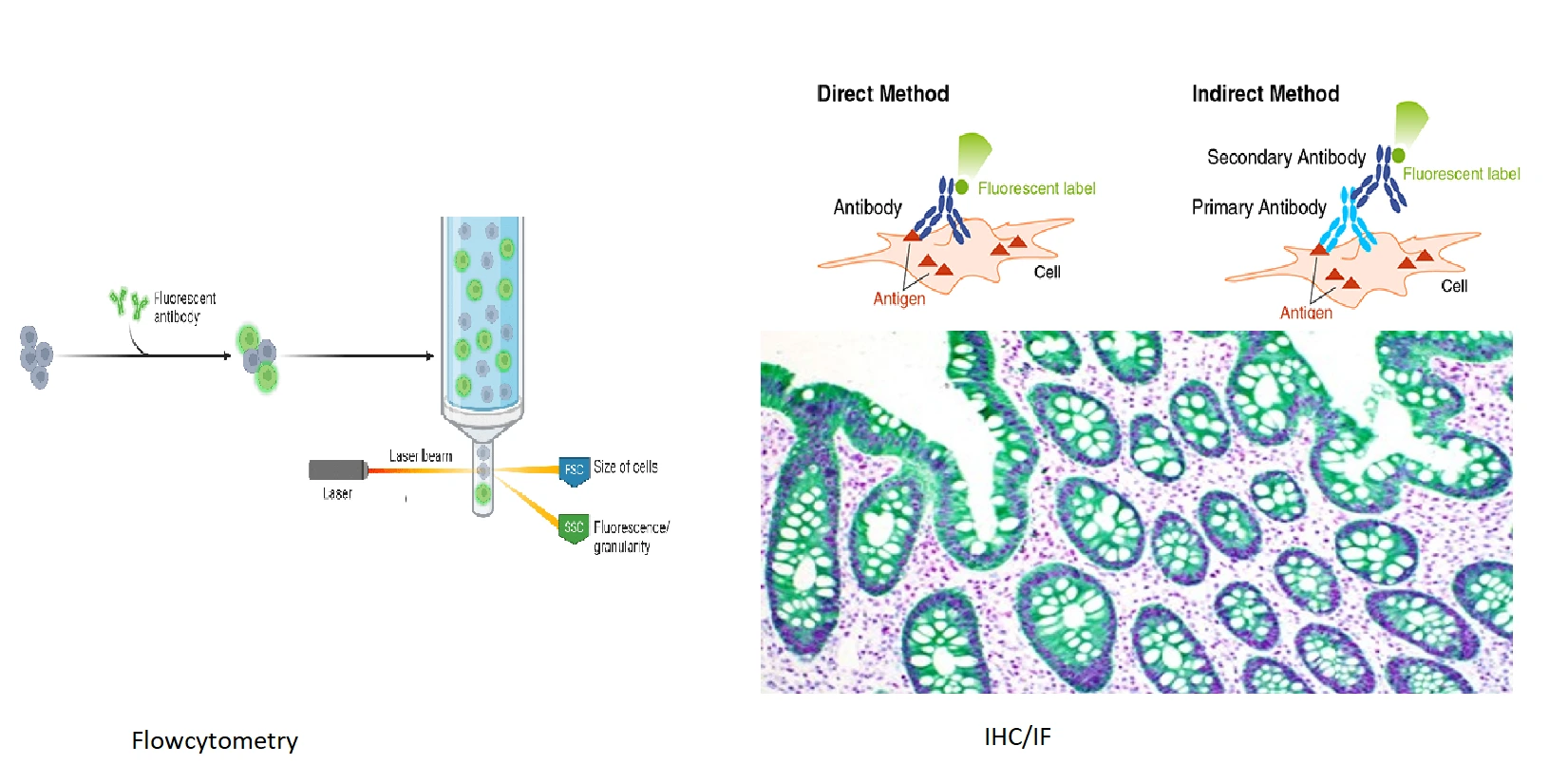
- Immunohistochemistry (IHC): IHC is used to visualize specific proteins or antigens in tissue sections. It relies on the specific binding of antibodies to their antigens.
- To prevent background noise, non-specific binding is blocked using buffers like serum or BSA.
- Visualization methods include attaching enzymes or fluorescent tags to antibodies, which produce color or light signals. IHC is critical for diagnosing diseases like cancer and studying the localization of biomarkers in tissues.
- Immunofluorescence is a microscopy technique where fluorescent dyes attached to antibodies help locate specific molecules in cells or tissues. There are two types:
- Primary Immunofluorescence: A single antibody directly labeled with a fluorescent dye binds to the target.
- Secondary Immunofluorescence: A secondary antibody with a dye binds to an unlabeled primary antibody, amplifying the signal.
- This method is used to study protein distribution and cellular structures like filaments or membranes.
- Flow Cytometry: Monoclonal antibodies are used in flow cytometry to analyze cells based on specific cell surface markers. This technique is commonly used in hematology and immunology to profile cells in a sample, such as identifying types of white blood cells.
- Lateral Flow Assays: Common in point-of-care diagnostics (e.g., pregnancy tests), mAbs are used in lateral flow assays to quickly detect specific pathogens or biomarkers in patient samples.
Difference between Monoclonal Antibodies and Polyclonal Antibodies for diagnostic uses
To understand the advantages of monoclonal antibodies (mAbs), let’s compare them with polyclonal antibodies:
| Polyclonal Antibodies | Monoclonal Antibodies (mAbs) |
| Produced by multiple B cells | Derived from a single B cell clone |
| Recognize multiple epitopes | Bind to a single, specific epitope |
| Variability between batches | High consistency and reproducibility |
| May cause cross-reactivity | High specificity, minimal non-specific binding |
| Limited applications in diagnostics | Ideal for diagnostics and therapeutics |
For diagnostic applications, precision and reproducibility are non-negotiable, which is why monoclonal antibodies are now the gold standard
Advantages of Monoclonal Antibodies as Diagnostic Reagents
- High Specificity: mAbs bind to a single epitope, reducing cross-reactivity and ensuring accurate results.
- Consistency and Reproducibility: Because mAbs are derived from a single clone of cells, they provide uniform and consistent diagnostic results across batches.
- Multiplexing: Multiple mAbs can be used together in a single assay to detect different biomarkers simultaneously, enhancing diagnostic capabilities.
- Customization: mAbs can be generated for almost any antigen, providing the flexibility to diagnose a wide range of diseases, including infectious diseases, cancers, and autoimmune disorders.
Challenges in Using Monoclonal Antibodies as Diagnostic Reagents
- Production Costs: The production of monoclonal antibodies can be expensive due to the complexity of hybridoma technology and the need for purified reagents.
- Limited Epitope Recognition: mAbs are highly specific to a single epitope, which may not always be present or accessible in all conditions, leading to false negatives in some cases.
- Immunogenicity: In some applications, especially in human diagnostics, the animal origin of mAbs can lead to immune responses in patients, especially if the antibodies are not humanized.
- Regulatory and Ethical Issues: The use of animals in mAb production raises ethical concerns, and the regulatory processes for approval of diagnostic mAbs can be lengthy and complex.
Examples of Diagnostic Reagents Using Monoclonal Antibodies
- Infectious Diseases: mAbs are used to detect pathogens such as viruses (e.g., HIV, hepatitis), bacteria (e.g., Salmonella, Mycobacterium tuberculosis), and parasites (e.g., Plasmodium falciparum).
- Example: HIV diagnosis often uses ELISA kits containing mAbs to detect the presence of HIV-specific antibodies or antigens.
- Cancer Diagnostics: mAbs are used to detect tumor-associated antigens such as HER2/neu in breast cancer, prostate-specific antigen (PSA) for prostate cancer, and CA125 for ovarian cancer.
- Example: Herceptin (trastuzumab) is a monoclonal antibody used both in cancer diagnosis and therapy, specifically targeting HER2-positive breast cancer.
- Autoimmune Diseases: Monoclonal antibodies are used to detect autoantibodies in diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and celiac disease.
- Pregnancy Tests: mAbs are used in home pregnancy tests to detect human chorionic gonadotropin (hCG), a hormone present in early pregnancy.
Future Directions in Monoclonal Antibody-Based Diagnostics
- Humanized and Fully Human mAbs: Advances in biotechnology are leading to the development of fully human mAbs that reduce the risk of immunogenicity and provide greater therapeutic and diagnostic potential.
- Nanobody Technology: Smaller antibody fragments, known as nanobodies, are being developed to overcome some of the limitations of conventional mAbs, such as poor tissue penetration.
- Point-of-Care Diagnostics: The future of monoclonal antibodies in diagnostics is leaning toward faster, more cost-effective point-of-care testing for conditions like infectious diseases, cancers, and genetic disorders.
- Multiplexed and Pooled Assays: The ability to test for multiple diseases or biomarkers simultaneously using multiplex assays is becoming more common, improving diagnostic efficiency.
Key Points on Monoclonal Antibody (mAb) Reagents in Drug Manufacturing:
1. mAb Reagents in Drug Substance Manufacturing
- Focus Areas:
- Biological safety (adventitious agents, impurities).
- Performance characteristics (binding specificity and avidity).
- Residual presence in the final product.
- Recommendations:
- Specify and test incoming mAb reagents for identity, binding activity, and contaminants.
- Monitor leachables and optimize purification to maintain product purity.
2. Purification of Drug Substances
- Testing:
- Affinity and specificity studies for target binding.
- Monitoring leachables (column lifespan factors like storage and regeneration solutions).
- Documentation:
- Data on column performance, stability, and microbial contamination must be submitted.
3. Comparability Requirements
- Changes in mAb suppliers or manufacturing processes require comparability testing for purity, performance, and biological safety.
4. Specifications for mAb Reagents
- Include a Certificate of Analysis (COA) with test results, expiration date, and a disclaimer (“Reagent use only; not for human use”).
- Tests for unconjugated mAbs: Identity (SDS-PAGE), purity (HPLC), protein concentration, target binding, microbial limits.
- Tests for mAbs on solid support: Particle size, binding capacity, mAb concentration, leachables, microbial limits.
5. Stability of mAb Reagents
- Real-time stability studies for unconjugated and conjugated mAbs should determine expiry dates.
- Ensure stability under storage, regeneration, and cleaning conditions.
This guidance helps ensure the safe and effective use of mAb reagents in drug manufacturing, particularly in purification processes.
Reference
Zola, H., Mohandas, A.P. and Krumbiegel, D. (2013). Monoclonal Antibodies: Diagnostic Uses. In eLS, (Ed.). https://doi.org/10.1002/9780470015902.a0002177.pub3
Tigabu Demlie, Endale Balcha and Haben Fesseha (2020).Monoclonal Antibody and its Diagnostic Application- Review, DOI: 10.26717/BJSTR.2020.30.004997
Deb, R. , Chakraborty, S. , Veeregowda, B. , Verma, A. , Tiwari, R. and Dhama, K. (2013) Monoclonal antibody and its use in the diagnosis of livestock diseases. Advances in Bioscience and Biotechnology, 4, 50-62. doi: 10.4236/abb.2013.44A008.